Automation of the Barnes maze requires reliable head tracking, an ability to cope with moveable zones and an easy way to end the test when the animal 'escapes'
ANY-maze fulfils all of these requirements and more - you'll find full details on the Benefits tab below.
On the other tabs you'll find videos of Barnes maze tests, recommended equipment and a list of results that are especially useful in this test.
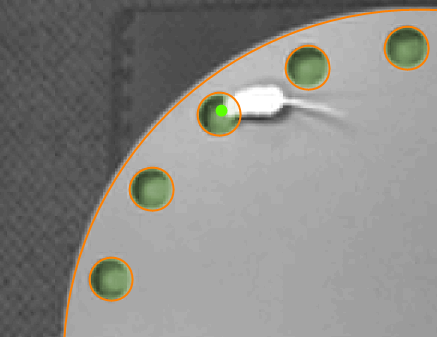
Head tracking
If you’re interested in determining how much the animal investigates the holes in the Barnes maze, then reliable head tracking is vital as the animal will typically only put its head into the hole.
The video on the right shows this in action, with ANY-maze accurately determining exactly when the animal is exploring the holes.
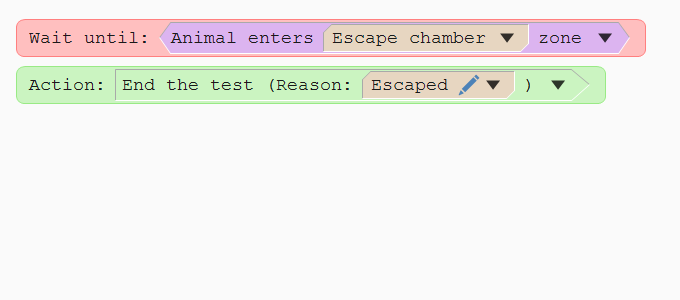
Ending the test when the animal 'escapes'
When the animal enters the escape hole it will disappear from the camera’s view. In ANY-maze you can define a hidden zone, which is where the software will consider the animal to be if it can’t find it anywhere else. So, to end the test when the animal escapes, you simply need to tell ANY-maze to wait until the animal enters this hidden zone – as the procedure on the right does.
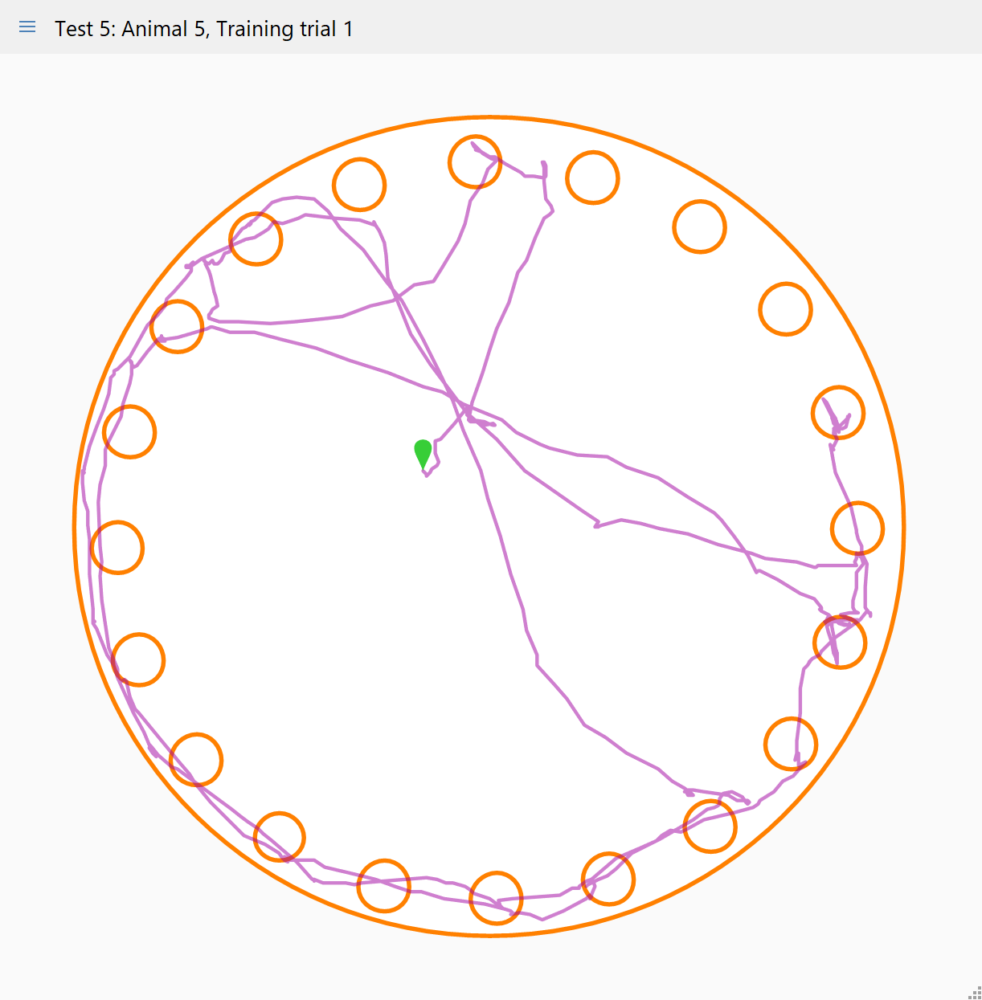
Viewing the animal's track
ANY-maze can plot the track the animal’s head took around the maze, which provides a great visual tool for both confirming the holes visited and for contrasting the behaviour of different animals.
Running multiple tests simultaneously
Running multiple Barnes mazes simultaneously is a great way to speed up the throughput in an experiment.
- Setting up multiple mazes is hardly any more work than setting up one, as most settings are automatically applied across all the apparatus.
- Tests in all the apparatus can be run independently, or you can control them together if you prefer.
Results
ANY-maze can provide literally hundreds of results for any test, but some of those that are commonly used in the Barnes maze include:
- Test duration (the test ends when the animal escapes)
- Latency to first visit to the escape hole
- Number of visits to non-escape holes
- Time investigating escape hole
- Time investigating non-escape holes
- Total distance travelled
Simultaneously tracking in four Barnes mazes
In this example ANY-maze is simultaneously tracking mice in four Barnes mazes. Note the thumbnails on the left, which you can use to select which apparatus are displayed – this is great when you want to focus on a specific test (note that the other tests continue be tracked, they’re just not displayed).
Mazes
We manufacture our own Barnes mazes, both for Rat and Mouse. The maze has a grey, non-reflective platform with 20 holes.
The legs can be detached which means the maze can be stored flat when not in use.

Cameras
USB Camera
The ANY-maze USB camera is an excellent choice for the Barnes maze. We recommend fitting this camera with a varifocal (zoom) lens, so you can simply mount the camera on the ceiling and then zoom in and out until the maze nicely fits the camera's view.
View more
Web cam
A webcam is usually a good, and inexpensive, alternative choice for the Barnes maze. If you intend to test in normal lighting conditions (>= 100 lux) and you can mount the camera far enough from the maze for it to see it all, then a webcam should work well.

Accessories
ANY‑maze Radio remote control
The ANY‑maze Radio remote control provides a convenient way to start the test as soon as the animal is in the maze. This remote works through walls and has 2 buttons, allowing you to control two apparatus independently.
View more
Scott G.A. et al (2021) Adult neurogenesis mediates forgetting of multiple types of memory in the rat. bioRxiv, https://doi.org/10.1186/s13041-021-00808-4
Wu, H. et al (2021) Polypharmacy Results in Functional Impairment in Mice: Novel Insights into Age and Sex Interactions. The Journals of gerontology, A, glab088
Monica, R.L. et al (2021) Characterization of nonmotor behavioral impairments and their neurochemical mechanisms in the MitoPark mouse model of progressive neurodegeneration in Parkinson’s disease. Experimental Neurology, 341, 113716
Wang, R. et al (2021) The Alteration of Brain Interstitial Fluid Drainage with Myelination Development. Aging and Disease, 10.14336
Evans A. et al (2021) Neurogenesis mediated plasticity is associated with reduced neuronal activity in CA1 during context fear memory retrieval. bioRxiv, https://doi.org/10.1101/2021.03.24.436893
Yang, L. et al (2021) Effects of prenatal photobiomodulation treatment on neonatal hypoxic ischemia in rat offspring Theranostics, 11(3),1269
Wickramasekara, R.N. et al (2021) Differential effects by sex with Kmt5b loss. Autism Research, https://doi.org/10.1002/aur.2516
Powers, K.G. et al (2021) Cell‐type specific knockout of peptidylglycine α‐amidating monooxygenase reveals specific behavioral roles in excitatory forebrain neurons and cardiomyocytes. Genes, Brain and Behavior, 20(2), e12699
Boscher, E. et al (2021) MicroRNA-138 Overexpression Alters Aβ42 Levels and Behavior in Wildtype Mice. Frontiers in Neuroscience, https://doi.org/10.3389/fnins.2020.591138
Lai, Z. et al (2021) Surgery Trauma Severity but not Anesthesia Length Contributes to Postoperative Cognitive Dysfunction in Mice. Journal of Alzheimer’s Disease, 80(1), 245
Tucker L.B. et al (2021) Hippocampal-Dependent Cognitive Dysfunction Following Repeated Diffuse Rotational Brain Injury in Male and Female Mice. Journal of Neurotrauma, https://doi.org/10.1089/neu.2021.0025
Karelina, K. et al (2021) Moderate Intensity Treadmill Exercise Increases Survival of Newborn Hippocampal Neurons and Improves Neurobehavioral Outcomes after Traumatic Brain Injury. Journal of Neurotrauma, http://doi.org/10.1089/neu.2020.7389
Zhong, J. et al (2020) A novel individual-based determination of postoperative cognitive dysfunction in mice. Aging and disease, 11(5), 1133
Nishida, S. et al (2020) Post-weaning folate deficiency induces a depression-like state via neuronal immaturity of the dentate gyrus in mice. Journal of Pharmacol. Sc., 143(2), 97
McMillan, P. et al (2020) Adult onset pan-neuronal human tau tubulin kinase 1 expression causes severe cerebellar neurodegeneration in mice. Acta Neuropathologica, 8, 200.
Lin, F. et al (2020) Attenuation of noisy environment-induced neuroinflammation and dysfunction of learning and memory by minocycline during perioperative period in mice. Brain research bulletin, 159, 16
R Bezbarua, F. et al (2020), Ethanolic Extracts of Dysphania ambrosioides Alleviates Scopolamine-Induced Amnesia in Experimental Animals.Phytomedicine and Alzheimer’s Disease, 1st edition
K.W. Sinkevicius et al. (2018) RNaseT2 knockout rats exhibit hippocampal neuropathology and deficits in memory; Disease Models & Mechanisms 2018: dmm.032631 doi: 10.1242/dmm.032631
A. Hojlan et al. (2018) Biochemical and cognitive effects of docosahexaenoic acid differ in a developmental and SorLA dependent manner; Behavioural Brain Research; 348:90-100
E.N. Dunn et al. (2018) Evaluating mice lacking serum carboxylesterase as a behavioral model for nerve agent intoxication; Toxicology Mechanisms and Methods 2018; 28(8): 563-572
Y. Yu et al. (2018) Facilitated AMPAR endocytosis causally contributes to the maternal sleep deprivation-induced impairments of synaptic plasticity and cognition in the offspring rats; Neuropharmacology 2018; 133:155-16
G. Bedse et al. (2018) Therapeutic endocannabinoid augmentation for mood and anxietydisorders: comparative profiling of FAAH, MAGL and dual inhibitors; Translational Psychiatry 2018; 8:92
HH MIAO et al. (2017) Ginsenoside Rb1 Attenuates Isoflurane/surgery-induced Cognitive Dysfunction via Inhibiting Neuroinflammation and Oxidative Stress. Biomed Environ Sci 2017; 30(5):363-372.
Lu Y, et al. (2016) Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol Aging. 2017 Jan; 49:165-182.
Zhao N, et al. (2016) Intranasal Delivery of a Caspase-1 Inhibitor in the Treatment of Global Cerebral Ischemia. Molecular neurobiology (2016): 1-17.
Lu Y, et al. (2016) Treadmill Exercise Exerts Neuroprotection and Regulates Microglial Polarization and Oxidative Stress in Streptozotocin-Induced Rat Model of Sporadic Alzheimer’s Disease. J Alzheimers Dis. 2017;56(4):1469-1484.
Fowler, S. W., et al. (2013) Effects of a metabotropic glutamate receptor 5 positive allosteric modulator, CDPPB, on spatial learning task performance in rodents. Neurobiology of learning and memory 99 (2013): 25-31.
Azzopardi, E., et al. (2013) Sensorimotor gating and spatial learning in α7-nicotinic receptor knockout mice. Genes, Brain and Behavior 12.4 (2013): 414-423.
Camara, Marie Lou, et al. (2013) TNF-α and its receptors modulate complex behaviours and neurotrophins in transgenic mice. Psychoneuroendocrinology 38.12 (2013): 3102-3114.
Rahn, Kristen A., et al. (2012) Inhibition of glutamate carboxypeptidase II (GCPII) activity as a treatment for cognitive impairment in multiple sclerosis. Proceedings of the National Academy of Sciences 109.49 (2012): 20101-20106.
Kovesdi, Erzsebet, et al. (2011) The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study. Front Neurosci 5.42.10 (2011): 3389.

 Setting up apparatus
Setting up apparatus Video capture & tracking
Video capture & tracking Observing behaviour
Observing behaviour Connecting equipment
Connecting equipment Automating complex tests
Automating complex tests Running tests
Running tests Results
Results Visualising data
Visualising data Analysis
Analysis Transferring data
Transferring data Open field
Open field Water-maze
Water-maze Y-maze
Y-maze Fear conditioning
Fear conditioning Novel object
Novel object Barnes maze
Barnes maze Radial arm maze
Radial arm maze Light/dark box
Light/dark box Operant conditioning
Operant conditioning Zebrafish
Zebrafish Computers
Computers Multifunction remote
Multifunction remote Accessories
Accessories Digital interface
Digital interface Optogenetic interface
Optogenetic interface Synchronisation interface
Synchronisation interface Relay interface
Relay interface Audio interface
Audio interface Touch interface
Touch interface Analogue interface
Analogue interface USB TTL cable
USB TTL cable Animal shocker
Animal shocker Components
Components Place preference
Place preference ANY-box
ANY-box T-maze
T-maze Zero maze
Zero maze Hole board
Hole board Sociability cage
Sociability cage OPAD
OPAD RAPC
RAPC Waterwheel forced swim test
Waterwheel forced swim test Thermal gradient ring
Thermal gradient ring Operon
Operon Activity Wheel
Activity Wheel Full ANY-maze licence
Full ANY-maze licence Other licence types
Other licence types Developing countries licence
Developing countries licence Contact support
Contact support Support Policy
Support Policy FAQs
FAQs Guides
Guides Downloads
Downloads Send us files
Send us files Activate a licence ID
Activate a licence ID Contact us
Contact us Blog
Blog About
About Testimonials
Testimonials Privacy Policy
Privacy Policy